Link between vitamin D deficiency, obesity and various diseases
Over the past three decades, obesity has been widespread worldwide, along with an increase in type 2 diabetes and hypovitaminosis D. If current trends continue, the World Obesity Federation estimates that more than one billion adults will be obese, and 2 .7 billion people will be overweight by 2025. Because obesity is associated with chronic diseases such as some cancers, cardiovascular disease and type 2 diabetes, the disease is of increasing importance. Recently, vitamin D deficiency in the development of obesity has attracted attention. It is clear that a deficiency of circulating vitamin D is associated with obesity and type 2 diabetes, and hypovitaminosis D and obesity result in such common diseases as type 2 diabetes, cardiovascular disease and some types of cancer. High vitamin D deficiency worldwide is generally the result of decreased production in the skin due to decreased sun exposure and decreased vitamin D intake. In areas where the sun does not shine as often, vitamin D is not photosynthesized properly, so European populations countries are commonly deficient in vitamin D. Vitamin D deficiency occurs even in parts of the world that are considered deserts and have long day lengths. It is well known that vitamin D is involved in bone mineralization, but recent studies have shown that hypovitaminosis D is responsible for more pathological conditions than previously thought. For example, metabolic syndrome, insulin resistance, and diabetes mellitus can potentially develop as a result of vitamin D deficiency.
Vitamin D
Today we're starting a long conversation on the topic of Vitamin D. Vitamin D is found in very few foods and is produced by our skin's exposure to ultraviolet rays. We will study all the variety of its different effects gradually and in doses, but for now we must consolidate eating behavior that helps prevent vitamin D deficiency.
“But we live in sunny Crimea!” - you say. Our latitudes are not the same. Ultraviolet rays are refracted in the atmosphere throughout almost all of Russia and do not have the expected synthesis of vitamin D in our skin.
There are 4 concepts in the meaning of vitamin D:
- Vitamin D deficiency - plasma level less than 20 ng/ml (50 nmol/l);
- Vitamin D deficiency - blood plasma level 20-30 ng/ml (50-75 nmol/l);
- Normal vitamin D levels are plasma levels of 30-100 ng/ml (75-250 nmol/l);
- Toxic vitamin D level is a plasma level above 150 ng/ml (above 325 nmol/l).
To prevent vitamin D deficiency, we should take 600-800 IU of this vitamin, and for its normal concentrations (prevention of vitamin D deficiency) 1500-2000 IU.
Pregnant women are recommended to receive at least 800-1200 IU per day. Let's see how much of this valuable vitamin is contained in food and how much wild salmon and fish oil you need to eat daily to provide yourself with vitamin D.
- Wild salmon - 600-1000 IU per 100 g
- Farmed salmon - 100-250 IU per 100 g
- Herring - 294-1676 IU per 100 g
- Som - 500 IU per 100 g
- Canned sardines - 300-600 IU per 100 g
- Canned mackerel - 250 IU per 100 g
- Canned tuna - 236 IU per 100 g
- Fish oil - 400-1000 IU per 1 tbsp. spoon
- Mushrooms irradiated with ultraviolet rays - 446 IU per 100 g
- Mushrooms not irradiated with ultraviolet rays - 10-100 IU per 100 g
- Butter - 52 IU per 100 g
- Milk - 2 IU per 100 g
- Milk fortified with vitamin D - 80-100 IU per glass
- Sour cream - 50 IU per 100 g
- Egg yolk – 20 IU per piece
- Cheese - 44 IU per 100 g
- Beef liver - 45-15 IU per 100 g
Vitamin D promotes calcium absorption in the intestines and maintains adequate levels of calcium and phosphate in the blood to strengthen bone tissue and prevent cramps.
It is also necessary for bone growth and proper metabolic processes in them (so that bone destruction does not occur faster than bone tissue restoration). Well, as we discussed earlier, vitamin D is used together with calcium for prevention as part of the complex treatment of osteoporosis.
That's all, in the broad sense of this vitamin in medicine. Just a small tip of the iceberg. Now let's expand our horizons:
- Skin – Reduces the risk of skin cancer from exposure to ultraviolet light.
- Hair – affects the renewal of hair follicles.
- Obesity and type 2 diabetes are much more common in people with vitamin D deficiency.
- Kidneys—vitamin D deficiency is a new factor in the progression of chronic kidney disease and renal failure.
- Malignant tumors - D prevents colon cancer.
- Heart - Low levels of vitamin D in humans are associated with adverse cardiovascular risk factors. D prevents the deposition of cholesterol plaques.
- Immune system - prevents autoimmune diseases (type 1 diabetes, multiple sclerosis, rheumatoid arthritis) and reduces the risk of infections (tuberculosis, ARVI, HIV, hepatitis C, etc.).
- Reproductive system - Vitamin D deficiency is associated with the risk of developing polycystic ovary syndrome. Vitamin D supplementation in men is associated with increased testosterone levels in the blood.
- Pregnancy - Vitamin D deficiency during pregnancy is associated with adverse pregnancy outcomes: increased risk of preeclampsia, infections, preterm birth, gestational diabetes.
Dementia - Research suggests that vitamin D may provide protection in older patients against neurodegenerative diseases such as Alzheimer's disease.
Causes of vitamin D deficiency. The geographical location of most of the Russian Federation in the northern latitude above the 35th parallel, in which, due to the more acute angle of incidence of sunlight and their dispersion in the atmosphere, from November to March the skin practically does not produce vitamin D, regardless of the amount of time a person spends in the sun. The lack of nutrients in the diet of a modern person is the result of inadequate intake of vitamin D from food, impaired absorption, increased need, inability to properly use it in the body, as well as its increased destruction.
Allergy to milk protein, lactose intolerance and vegetarianism play a significant role in the development of vitamin D deficiency. In earlier posts, we already introduced you to foods rich in vitamin D. I can see how you happily eat a spoonful of fish oil in the morning or fight on the banks of a mountain river with a bear for the territory of catching wild salmon.
Another factor in the widespread D deficiency is the obesity epidemic. An increase in the mass of adipose tissue leads to the binding of D and its increased deposition in the subcutaneous fat and inaccessibility to the central bloodstream.
Significant causes of vitamin D deficiency are decreased digestion of fats due to malabsorption in the intestine, including in patients after bariatric surgery, as well as loss of vitamin D in the urine in combination with its binding protein in renal failure.
Some medications have a significant effect on the metabolism of vitamin D in the body and are associated with its increased degradation into inactive forms (glucocorticoids, antiretrovirals, antifungals, cholestyramine, antiepileptic drugs). About drugs for the treatment of vitamin D insufficiency and deficiency. As before, I will not describe any recommendations on drug doses and treatment regimens. Vitamin D is not for self-medication; its concentrations are likely to have a toxic effect.
The recommended drug for the treatment of vitamin D deficiency is colecalciferol (D3). Two forms of the drug are registered in Russia - an oil solution of Vigantol and an aqueous solution of Aquadetrim. One drop of these solutions contains 500 IU of the drug.
Colecalciferol is the safest and easiest way to correct vitamin D deficiency. In the Russian Federation, Japan and some other countries, active metabolites of vitamin D and their analogues - calcitriol and alfacalcidol - are often used. Due to the significantly higher cost and the need to monitor blood calcium and urine calcium, the use of these drugs is not recommended in cases where effective use of native vitamin D (colecalciferol) is possible.
To monitor the effectiveness of prescribed doses of active metabolites of vitamin D and their analogues, it is necessary to use the concentration of total and/or ionized calcium, parathyroid hormone in the blood (the level of vitamin D-25-OH will be uninformative when treating with these forms).
Research on the relationship between body mass index and vitamin D levels
Danish scientists associated with Aarhus University found 55 previously published studies in which researchers measured both vitamin D levels and BMI in large groups of people. The Danes summarized the results of these studies and analyzed them again.
Of the 45 studies, data on people without diabetes was selected and pooled. The researchers then divided the data into three groups: the first group of data was for people with a BMI of 18-25, the second for people with a BMI of 25-30, and the third for people with a BMI over 30 (who were obese).
In all groups, the level of vitamin D in the blood of the subjects was below optimal, but in the group with the highest BMI the relationship was the strongest.
The researchers did the same with data from people with diabetes and came to the same conclusion. In this group, the association between vitamin D and BMI was even stronger than in the group without diabetes. Therefore, it is suggested that individuals with a BMI greater than 30 should take vitamin D more frequently and in higher doses.
Conclusion
This review provides evidence that serum vitamin D levels are inversely associated with BMI in diabetic and nondiabetic patients. The strong inverse correlation between vitamin D deficiency and BMI may be due to the association of vitamin D deficiency and obesity with a number of diseases. The coexistence of these two factors may be important for the development of certain pathological conditions, for example, type 2 diabetes mellitus is closely associated with obesity and vitamin D deficiency. It has previously been noted that the synergistic effect of obesity and vitamin D deficiency may contribute to the development of insulin resistance.
Epidemiological studies like this show only associations, but not every association is a cause-and-effect relationship. However, since other studies show that vitamin D affects how muscles metabolize fat and energy, it is possible that low vitamin D levels are indeed a factor in obesity.
Excess
Usually, when menopause or an interesting situation occurs, women begin to actively take various vitamin-mineral complexes and monovitamins. However, you need to know that D3 is a fat-soluble compound, accumulates in the body as a depot in adipose tissue, and is excreted very slowly.
With prolonged use of dietary supplements over many days, the vitamin begins to exhibit toxic properties. This usually occurs when the level reaches 10,000 IU or more. It is significant that an overdose is possible only with oral administration; prolonged exposure to the sun does not affect this in any way.
Sometimes symptoms of hypervitaminosis can appear against the background of frequent preventive courses in children. After sensitization of the body, hypersensitivity and individual intolerance occur.
Hypervitaminosis is divided into two stages. Initially, a person feels weak and nauseous, and stool disorders develop - from diarrhea to retention. Appetite disappears, the patient begins to lose weight sharply, and experiences headaches and muscle and joint pain. Increased urge to urinate
The second stage is characterized by dehydration, convulsive and febrile syndromes. Calcium is gradually deposited on the walls of blood vessels and in the tissues of parenchymal organs. Calcinosis provokes atherosclerosis. In the most severe cases, a coma occurs.
Excess calciferol is fraught with the appearance of calcium in the blood and urine - calciuria and calcemia. In turn, this causes arrhythmia, hypertension, infertility, and early menopause.
Therefore, if you intend to take dietary supplements for a long time, be sure to take a blood test and consult with your doctor.
The relationship between vitamin D content, the amount of muscle mass and the volume of adipose tissue
The relationship between the level of vitamin D in the blood and the volume of adipose tissue has been proven. The more vitamin D you have, the less fat you have and the more muscle mass you have. This conclusion was reached by researchers from Mahidol University in Thailand. The study involved 163 overweight people of different genders.
To measure the content of muscle mass and volumes of adipose tissue, the bioelectrical impedance method was used. The amount of 25-hydroxyvitamin D was measured by a complete blood count. Despite the fact that Thailand is a tropical country with a lot of sunny days, most of the participants in the experiment showed low levels of vitamin D in the blood.
Previous studies have found that obesity is associated with low levels of vitamin D. This is partly because as weight increases, there is no corresponding increase in skin volume. Thus, the amount of skin that could absorb sunlight and help the body produce vitamin D cannot keep up with the increase in fat tissue.
Similarly, the study participants showed that higher levels of vitamin D were found in people with less body fat and more muscle mass. Laboratory studies have shown that muscle cells produce less myostatin when exposed to vitamin D. Vitamin D-deficient muscle cells have also been found to break down muscle tissue more quickly.
Statistical calculations have shown that a large amount of adipose tissue has a negative effect on the amount of muscle mass. The tables below show the direct relationship between vitamin D content, the amount of muscle mass and the amount of fat tissue.
It can be concluded that increasing the level of vitamin D in the blood leads to an increase in muscle mass.
| Fat percentage | Vitamin D content in blood, ng/ml |
| 20 | 26 |
| 30 | 25 |
| 40 | 24 |
| 50 | 22 |
| 60 | 21 |
| Amount of muscle tissue in kilograms | Vitamin D content in blood, ng/ml |
| 10 | 21 |
| 20 | 22 |
| 30 | 23 |
| 40 | 24 |
| 50 | 25 |
| 60 | 26 |
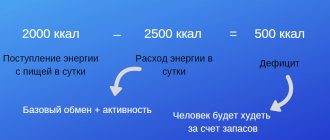
Effect on weight gain
Endocrinologists say that this substance is essentially a hormone, not a vitamin. Receptors for it are located in the kidneys, pancreas, and in the cells of the nervous and immune systems. Therefore, the component is so important for all processes occurring in the body. Including for weight loss.
Research on how vitamin affects weight gain or loss began not so long ago. However, experts have already found out that when there is a lack of substance, cells do not receive a signal from receptors to burn fat. If there is enough of it in the tissues, weight loss occurs more effectively.
A study of more than 4,600 older women found that higher vitamin D levels were associated with less weight gain between doctor visits over the course of a 4.5-year study. ()
The effect of vitamin D on increasing lean body mass
If healthy, normal, non-athletic people take a moderate dose of vitamin D3 every day for a year, their lean body mass can increase by half a pound. Chinese scientists from the Health Science Center of Xi'an Jiaotong University came to this conclusion in a real-life human study that was not sponsored by the supplement manufacturer.
Gain Lean Mass with Vitamin D: Study
This study aimed to determine whether 1 year of vitamin D3 supplementation had a direct effect on body composition and fitness in healthy adults. The researchers recruited 95 healthy people aged 20-69 years to participate. 47 of them received a placebo every day for one year, another group of 48 people received 420 IU of vitamin D3 every day for the same period. This dose is 10.5 micrograms of vitamin D3 per day. This is approximately the dose that the Dutch Nutrition Center recommends for healthy people who rarely go outside.
Research results
After one year of supplementation, the concentrations of hydroxy-vitamin D3 and dihydroxy-vitamin D3 in the blood of subjects in the supplement group were significantly higher than those in the placebo group. The increase in hydroxyvitamin D3 (25(OH)D) and dihydroxyvitamin D3 (1.25[OH]2D) concentrations as a result of supplementation occurred by approximately 11.2 ± 9.2 ng/ml and 7.0 ± 7 .8 pg/ml respectively.
When the researchers measured the subjects' blood pressure, fat mass, strength and endurance after a year of supplementation, they saw no differences between the placebo group and the experimental group. But when they looked at lean mass (body mass without fat), they found a significant difference between the groups. Lean body mass significantly increased from 43.8 ± 9.6 to 44.3 ± 9.8 kg in the vitamin D group, while
as in the placebo group, no changes were observed (from 42.6 ± 8.9 to 42.4 ± 8.9 kg) after 1 year of intervention. Lean mass primarily consists of skeletal muscle tissue, but also includes internal organ mass and bone mass.
Conclusion from the study
“The present study showed that treatment with vitamin D 420 IU/day for 1 year did not appear to affect body fat, muscle strength, or cardiorespiratory performance, while lean body mass was improved,” the researchers wrote.
Why you need a comprehensive vitamin D test
Even if you take the Vitamin D25-OH test, you will not see the full picture of the vitamin’s effect on the body. Conversely, if you determine what level of calcium, phosphorus or magnesium you have in your body, you will need to find out the possible reasons for these levels. This will allow you to prescribe the most effective treatment.
Therefore, medical experts of the online laboratory Lab4U have developed a special complex - Examination for vitamin D and calcium deficiency. It will allow you to comprehensively study the effect of vitamin D on phosphorus-calcium metabolism and the body as a whole, as well as prevent possible negative consequences of a lack or excess of vitamin D.
Get tested and bring the results to your doctor. If necessary, he will prescribe treatment for you.
You can take a complex test for vitamin D and calcium deficiency with a 50% discount. The analysis will be ready within 1 day. You will receive the results by email as soon as they are ready.
Link between vitamin D deficiency and chronic pain
Vitamin D, a fat-soluble vitamin found in few natural food sources, is synthesized in human skin after sun exposure. Up to 80% of patients in healthcare settings are deficient in vitamin D, which is believed to contribute to a wide range of health problems. Vitamin D has long been used in combination with calcium to improve bone health and reduce the risk of fractures.
Taking vitamin D has been linked to the prevention of high blood pressure, cancer and other diseases. Recent research also points to a possible link between vitamin D deficiency and chronic pain. There are two forms of vitamin D used in humans—ergocalciferol (vitamin D2) and cholecalciferol vitamin (D3). While D2 is primarily synthesized by plants, sunlight can promote the synthesis of D3 in human skin. Since few foods naturally contain vitamin D, skin synthesis is typically the main source.
Research
A number of studies have revealed evidence that vitamin D promotes weight loss.
Slim & plump
Overweight people have extremely low levels of vitamin D, while thin people have high levels.
Abdominal obesity
In people with abdominal obesity, a large accumulation of vitamin D was found in the fat folds. It turned out that for accelerated weight loss they need:
- Play sports with an emphasis on exercises for the waist and abdomen.
- Take increased dosages of vitamin D, since more than 40% of this amount goes into abdominal fat.
The abdominal cavity needs to be oversaturated with this substance so that it begins to perform its immediate functions: burn adipocytes and build muscle mass. Therefore, at first the process of losing weight will be slow, but once the desired result is achieved, its speed will increase significantly.
Iranian scientists have proven this through research. One group of overweight women was given 1000 IU of vitamin D per day, the other was given a placebo. After 3 months, in the former, the amount of abdominal fat decreased by an average of 2.5 to 3 kg, in the latter, no changes occurred.
Muscle mass
Experimental rats that were fed sugar and fatty foods, but at the same time given increased doses of vitamin D in combination with calcium, did not gain fat, but lost weight against the background of an increase in muscle mass.
Diabetes
A study was conducted at the University of Missouri that found that supplemental vitamin D intake prevents the development of diabetes, which in 80% of cases is associated with excess weight.
Appetite
Vitamin D affects areas of the brain responsible for feelings of satiety and hunger. If it is not enough, the signal about the onset of satiety during a meal is not received, and the person overeats.
Waist reduction
The University of Milan experiment involved more than 400 people suffering from excess weight and obesity. Everyone was on a diet. The first group did not take any medications. The second is 25 doses of vitamin D per month. Third - 100 dosages. Six months later, waist size decreased on average in group I subjects by 3.2 cm, in group II by 4 cm, and in group III by 5.5 cm.
The effectiveness of vitamin D3
There is currently debate about the role of vitamin D deficiency in pain. While some studies have shown no correlation between pain concentrations and vitamin D, others have found an association of increased incidence of chronic pain with serum vitamin D concentrations along with increased incidence of chronic and nonspecific musculoskeletal pain.
These patients often report a lower quality of life. The type of pain associated with vitamin D deficiency varies, but it usually manifests as chronic musculoskeletal or generalized bone pain. The correlation between serum vitamin D concentrations and pain has been studied in various types of pain, including chronic pain, musculoskeletal pain, rheumatoid arthritis,
Obesity-related back pain and headaches. The mechanism of action of vitamin D for pain symptoms depends largely on the type of pain. Although it is thought that vitamin D may promote the release of inflammatory cytokines in rheumatoid arthritis, for muscle pain it is thought that vitamin D may reduce the sensitivity of nerve fibers in the muscles. Observational studies, placebo-controlled studies, and meta-analyses have been conducted to examine the correlation between vitamin D concentrations and pain symptoms.
In an epidemiological study by Hirani and colleagues, vitamin D concentrations and pain symptoms were examined in 1659 men aged >70 years. The 12-item Mini-Health Survey assessed pain levels experienced during the previous 4 weeks, and the Physical Activity Scale for Older Adults was used to assess each patient's physical activity level.
Patients with low vitamin D concentrations had higher rates of both obsessive and chronic pain, but statistical significance was lost after adjustment for covariates. The incidence of chronic pain remained significant in patients with the lowest quartile of vitamin D concentrations compared with the incidence in those in the highest quartile, even after adjustment for covariates. [5]
A retrospective observational study by Matosian-Motley and colleagues reviewed the records of 414 adults admitted to a rehabilitation facility over a 1-year period. The concentration of vitamin D in the blood serum of patients was recorded, as well as pain intensity scores on a visual analogue scale (VAS) upon admission. Patients with vitamin D concentrations <20 ng/mL had higher odds of nonspecific musculoskeletal pain compared with patients with serum vitamin D concentrations >20 ng/mL. Similarly, patients with serum vitamin D concentrations <30 ng/mL had higher odds of nonspecific musculoskeletal pain compared with patients with serum vitamin D concentrations >30 ng/mL.
Adjustment for supplemental vitamin D use and environmental season at presentation did not significantly change the results. [6] A prospective, randomized, double-blind, placebo-controlled study by Knutsen and colleagues examined changes in pain from baseline with vitamin D supplementation. Patients received vitamin D 10 mcg or 25 mcg or placebo for 16 weeks. Participants were assessed using the VAS analogue scale at baseline and again after 16 weeks to determine pain intensity and location. Serum vitamin D concentrations were also measured. Compared with baseline, there were no significant differences in pain severity, pain location, or pain medication use between the vitamin D and placebo groups at 16 weeks. [7]
Another prospective study by Le Goaziu and colleagues examined the effect of vitamin D supplementation on pain in 49 patients who presented to their physician with complaints of diffuse musculoskeletal pain and had low serum vitamin D concentrations. Patients were prescribed high doses of vitamin D (400,000–600,000 IU) depending on the severity of vitamin D deficiency.
Pain was assessed (depending on location, duration, intensity and use of analgesics) at baseline and after the intervention. At follow-up, mean patient pain scores decreased from 5.1 to 2.8 points, and the proportion of patients self-reporting analgesic use decreased from 20% to 12.2%. [8]
The use of fortified foods has also been investigated as a means of supplementing vitamin D. Kostan and colleagues administered a daily serving of bread containing 125 mcg of vitamin D3 to up to 45 nursing home residents. After 12 months, serum vitamin D3 concentrations were significantly improved, as were pain scores and activities of daily living. [9]
While most studies have examined the relationship between vitamin D concentrations and pain, some studies have also examined vitamin D concentrations and surrogate markers of inflammation and pain. A retrospective analysis by Hong and colleagues assessed the correlation between vitamin D concentrations and inflammatory cytokines and disease status in patients with rheumatoid arthritis. Patients with lower vitamin D concentrations had significantly higher incidences of disease symptoms such as swollen joints, joint stiffness and joint pain. In addition, vitamin D concentrations were negatively associated with the presence of inflammatory cytokines, particularly interleukin-17 and interleukin-23. [10]
Vitamin D increased: what to do
High levels of vitamin D can lead to anorexia, weight loss, increased urine output, and cardiac arrhythmia. Remember that vitamin D can increase blood calcium levels. This will lead to calcification of blood vessels and tissues. This can cause damage to the heart, blood vessels and kidneys. Therefore, during the period of taking additional vitamin D, we recommend getting tested at least once every 6 months.
It is recommended to check your vitamin D level before starting its correction and after treatment. This will allow you to monitor changes in levels over time and evaluate the effectiveness of treatment. At Lab4U you can take a comprehensive test for vitamin D and calcium deficiency with a 50% discount . You will receive the results by email. They will also be available in your personal account. Now it is very easy to observe the dynamics of changes in the body’s indicator.
According to research by the Russian Association of Endocrinologists, an excess is only possible with an overdose of vitamin D - consumed in doses exceeding those recommended by the attending physician.
How to take vitamin D3
Two weeks of supplemental vitamin D: improvement, fatigue, blood pressure and cortisol levels.
If you give a group of healthy people 50 mg of vitamin D3 every day for two weeks, their endurance levels will increase significantly. They will feel less tired during exercise and cover longer distances, and their blood pressure will drop, as will the concentration of cortisol in the body. Scottish scientists from Queen Margaret University report this in the journal Therapeutic Advances in Endocrinology and Metabolism.
Vitamin D supplementation study
Researchers gave 9 healthy people 2,000 units (equal to 50 mg) of vitamin D3 every day for 14 days. Six people were given a placebo. The researchers conducted various tests on the participants before and after the period of vitamin D3 supplementation.
results
Before and after the vitamin D supplementation period, participants were required to exercise on a stationary bicycle for 20 minutes. At the end of the vitamin supplementation period, participants taking vitamin D walked a greater distance than participants in the placebo group.
Also, participants taking vitamin D felt less tired immediately after completing a stationary bicycle session than participants in the placebo group.
Taking vitamin D also lowered both systolic and diastolic blood pressure levels, both while participants were resting [first picture below] and during a cycle ergometer session [second picture].
In the left picture below you can see that taking vitamin D reduced the concentration of cortisol in the urine.
The picture on the right shows how vitamin D lowered the cortisol:cortisone ratio in the participants' urine.
Mechanism
“Vitamin D exerts antihypertensive effects by suppressing the renin-angiotensin-aldosterone system (RAS), and the relationship between vitamin D levels and renin was first established in 1986,” the researchers report. “Ras is a vital regulator of blood pressure through renin activity, where renin binds angiotensin I and angiotensin II together, and once it binds to the receptor it has a regulating effect on blood pressure. "Inappropriate stimulation of RAS leads to hypertension, which suggests that suppressing RAS with vitamin D may lower blood pressure."
“Decreased cortisol and the urinary cortisol:cortisone ratio indicate decreased levels of stress hormones, which may occur due to decreased activity of 11-beta-HSD1 (the enzyme responsible for converting cortisone to its active form, cortisol).”
Sources
- Body Mass Index, Vitamin D, and Type 2 Diabetes: A Systematic Review and Meta-Analysis. Shamaila Rafiq and Per Bendix Jeppesen. Nutrients 2021, 10(9), 1182;
- Nutrition 10.1016/j.nut.2014.11.011.
- Effect of Vitamin D Supplementation on Body Composition and Physical Fitness in Healthy Adults: A Double-Blind, Randomized Controlled Trial
- Sun X., Tanisawa K., Zhang Y., Ito T., Oshima S., Higuchi M., Cao Z. Ann Nutr Metab. 2019;1-7.
- Hirani V, Blyth FM, Naganathan V. Active vitamin D (1.25 dihydroxyvitamin D) is associated with chronic pain in older Australian men: The Concord Health and Ageing in Men Project. J Gerontol A Biol Sci Med Sci. August 7, 2014
- Matossian-Motley DL, Drake DA, Samimi JS, et al. Association between serum 25(OH)D level and nonspecific musculoskeletal pain in acute rehabilitation unit patients. JPEN J Parenter Enteral Nutr. October 14, 2014
- Knutsen KV, Madar AA, Brekke M, et al. Effect of vitamin D on musculoskeletal pain and headache: a randomized, double-blind, placebo-controlled trial among adult ethnic minorities in Norway. Pain. 2014;155:2591-2598.
- Le Goaziou MF, Kellou N, Flori M, et al. Vitamin D supplementation for diffuse musculoskeletal pain: results of a before-and-after study. Eur J Gen Pract. 2014;20:3-9.
- Costan AR, Vulpoi C, Mocanu V, et al. Vitamin D fortified bread improves pain and physical function domains of quality of life in nursing home residents. J Med Food. 2014;17:625-631.
- Hong Q, Xu J, Xu S, et al. Associations between serum 25-hydroxyvitamin D and disease activity, inflammatory cytokines and bone loss in patients with rheumatoid arthritis. Rheumatology (Oxford). 2014;53:1994-2001.
Vitamin D deficiency in obese children - who is at highest risk?
The level of human health is determined by a large number of internal and external factors, among which the prevalence in the population of a number of disorders and pathologies that are epidemic or endemic in nature plays a major role. The latter fully include overweight and obesity, as well as a decrease in the supply of vitamin D. Research in recent years has shown both the progressive growth of this group of pathologies, as well as its significant and not yet fully studied influence on the growth of metabolic, cardiovascular, immune, and oncological diseases. and many other diseases, as well as negative genome editing, which means the manifestation of the above-mentioned disorders in subsequent generations [1–3].
The widespread prevalence of reduced vitamin D levels, due to both the geographical location of the Russian Federation and other factors, affects, according to various authors, up to 90% of the population [4–6]. Vitamin D deficiency is as widespread among children as among adults [6–8]. The largest currently in the pediatric population of the Russian Federation is a multicenter study conducted by I. N. Zakharova et al. in 2013–2017, which showed that only 34% of young children and 5.2% of schoolchildren aged 11–17 years have normal vitamin D levels [9–12].
In recent years, the attention of researchers has begun to be attracted by the fact that vitamin D deficiency often combines with metabolic disorders in both adults and children [13–14], however, the geographical place of residence does not always have a significant effect on the level of 25(OH)D in the blood [15–14]. 21]. Thus, the results of a number of studies have shown that low levels of vitamin D in blood serum are most often observed in patients with obesity, type 2 diabetes mellitus (DM) [22–24], and dyslipidemia [25–26].
Given the fact that metabolic disorders are increasing in both adults and children, most experts are inclined to believe that a normal level of vitamin D is necessary to prevent the development of metabolic disorders.
Design, material and research methods
The study involved 127 children with primary exogenous constitutional obesity (main group), permanently residing in St. Petersburg and the Leningrad region, meeting the inclusion criteria: age 7–17 years, obesity, according to WHO criteria and federal recommendations of the Russian Federation (mass index body (BMI) ≥ +2 SDS for a given gender and age), absence of signs of acute disease or exacerbation of chronic disease at the time of inclusion in the study, absence of taking vitamin D supplements for at least one month before inclusion in the study. Exclusion criteria from the main group: obesity due to other endocrine diseases (hypothyroidism, hypercortisolism, hypopituitarism and other types), obesity due to injuries of the hypothalamic-pituitary region, obesity due to genetic syndromes (Prader-Willi syndrome, Bardet-Biedl, Down and others), the presence chronic diseases of the digestive tract, liver and kidneys.
The control group (n = 64) consisted of children and adolescents without obesity (BMI < +1 SDS for a given gender and age). The main group and the control group were comparable in age (average age 12.8 years and 13.0 years, respectively), stage of sexual development (22% and 21% of children in the groups had not started puberty, among adolescents in the rest of the groups - 78% and 79%, respectively - Tanner stages of puberty II–IV were recorded), gender composition (52% and 67% of those examined were male). All children and adolescents included in the study were examined at the clinical base of the Federal State Budgetary Institution National Medical Research Center named after. V. A. Almazova of the Ministry of Health of the Russian Federation in the period 2012–2016.
Within the obesity group (main group), 2 comparison subgroups were identified depending on the SDS BMI values: > +2 < +3 SDS BMI was considered milder obesity (1st subgroup, n = 75), ≥ +3 SDS BMI - progressive and more pronounced (2nd subgroup, n = 52). To assess the contribution of puberty, children and adolescents of the main group and the control group were divided according to the presence of the onset of puberty: the pre-puberty group (Tanner I) and the group with the start and progression of puberty (Tanner II–IV).
The anthropometric examination included height measurement using a Diakoms floor stadiometer; measuring body weight using medical floor electronic scales of the VEM-150 series (Mass-K). Calculation of BMI using the formula [weight (kg)/height (m2)]. BMI was assessed according to WHO standards and federal recommendations of the Russian Federation, with an assessment of the standard deviation SDS. Sexual development was assessed according to Tanner stages. Waist circumference (WC) was measured using a non-tensile measuring tape at the midpoint between the lower edge of the last palpable rib and the upper part of the iliac crest, according to WHO recommendations. Hip circumference (HC) was measured using a non-stretch tape around the widest part of the buttocks, keeping the tape parallel to the floor, also according to WHO recommendations. Clinical assessment of the presence of abdominal obesity was carried out by identifying children with WC greater than the 90th percentile for a given gender and age.
Instrumental examination: the amount and distribution of adipose tissue in the body was assessed using dual-energy X-ray absorptiometry (DXA) using the Lunar Prodigy device (USA) in the Total Body Composition scanning mode, radiation dose 0.0003 mGy. The total amount of body fat (TBF, kg), the percentage of android (A%) and gynoid (G%) fat and their ratio (A/G), the percentage of total body fat (W%) and tissue weight and organs without fat content (BZ, g). Based on the data on the total amount of fat, the fat mass index (FMI) was calculated using the formula: [FML (kg)/height (m2)].
The laboratory examination included an assessment of vitamin D status, an assessment of carbohydrate and lipid metabolism, the level of leptin, adiponectin and was carried out at the Central Clinical Clinical Laboratory of the Federal State Budgetary Institution National Medical Research Center named after. V. A. Almazova. Blood sampling was carried out on an empty stomach in the morning during the initial examination, 3 months after treatment with cholecalciferol (in the 1st and 2nd subgroups of the main group) and 6 months after the end of taking cholecalciferol (1st subgroup of the main group). The level of 25(OH)D was determined using the chemiluminescence method (Abbott Architect 8000 analyzer). The results were assessed in accordance with the recommendations of the European Society of Endocrinology (2011): vitamin D deficiency - 25(OH)D less than 20 ng/ml (less than 50 nmol/l); vitamin D deficiency - 25(OH)D 21–29 ng/ml (51–75 nmol/l); normal vitamin D content is 25(OH)D 30–100 ng/ml (76–250 nmol/l). A 25(OH)D content of more than 100 ng/ml (more than 250 nmol/l) was regarded as excess vitamin D, according to the recommendations of the European Society of Endocrinology. Glycated hemoglobin (HbA1c) was determined in unfrozen whole blood using a Bio-Rad D10 high-performance liquid chromatograph.
Plasma glucose levels were assessed using the glucose oxidase method (Abbott Architect 8000 analyzer). The subjects underwent a standard oral glucose tolerance test (OGTT, glucose load 1.75 g/kg, not more than 75 g) with determination of fasting glucose levels (glucose 0') and 120 minutes after glucose load (glucose 120'). Impaired fasting blood glucose (IFG) was diagnosed when fasting blood glucose was >5.6 mmol/L; Impaired carbohydrate tolerance (ICT) - with a glycemic level after 120 minutes > 7.8 mmol/l. The insulin content in blood serum was assessed using the enzyme immunoassay method (Cobas e411 analyzer). The index of insulin resistance (HOMA-IR) was calculated using the formula: fasting insulin (pmol/L) × fasting glucose (mmol/L)/155. Values less than 3.2 were taken as the normative HOMA-IR indicator.
The content of total cholesterol (TC), triglycerides (TG), high-density lipoproteins (HDL) and low-density lipoproteins (LDL) was studied using the enzyme immunoassay method (Cobas Integra 400 analyzer). The content of adipocytokines (leptin and adiponectin) was studied using a manual tablet determination using the enzyme immunoassay method using a set of HumanAdiponectin ELISA reagents (BioVendor). The obtained data were processed using the Statistica 7.0 for Windows (StatSoft) software system and in the Microsoft Office 2021 environment.
Research results
Features of obesity and body composition in children and adolescents
Significant risks of severe obesity with BMI ≥ +3 SDS, according to our data, were obesity in parents and the onset of obesity at an early age - in the first 3 years of life (OR, respectively, 3.3 and 3.7; p < 0.05) (Table 1).
The structure of the study included an in-depth study of the amount, distribution of fat and changes in these parameters over time, with the progression of obesity in children and adolescents, using an assessment of body composition using the DXA method. Currently, DXA remains the gold standard for assessing body composition and provides more accurate information about fat mass and its distribution. In the adult population, using this method, reference values of IMI for men and women have already been established: 4–6 kg/m2 for men and 5.0–8.9 kg/m2 for women [27]. In the pediatric population, such reference data are currently not available; local studies are being conducted that indicate different standards. Thus, according to the data of EW Demerathr et al., 2013 [28], in regional IMF percentile standards, the 90th percentile of the British population of children corresponded to the 75th percentile of the American population, which indicated significant differences in the reference values for the amount of adipose tissue in the studied populations children (Fig. 1).
The study also used the DXA method and characterized the body composition features of the examined children in St. Petersburg. The median IMI of children and adolescents was 12.9 kg/m2 [10.8–14.15], which was 1.5–2 times higher than the upper reference range for adults, and was beyond the threshold (90th percentile) IMI values for children for both European and American populations (Fig. 1). The median values of IMI among boys and girls were not statistically different and amounted to 12.6 kg/m2 [10.2–14.8] in boys and 14.5 kg/m2 [11.9–18.8] in girls (p = 0.09).
When conducting a correlation analysis, a strong direct connection between BMI and IMI was established, which allows us to assert that when diagnosing obesity in children, based on BMI values, it is the fat component that makes the most significant contribution; Moreover, the progression of BMI is accompanied by a unidirectional increase in IMI, which, in turn, confirms the increase in body weight in obese children precisely due to the fat component. The amount of fat of android localization has a direct significant correlation with the total amount of adipose tissue and the WC value; therefore, with the progression of obesity, the amount of fat of metabolically unfavorable android localization increases most significantly, which, in turn, is clinically reflected by an increase in waist circumference, the measurement of which can and should be used as an assessment of clinical risks of comorbidity in obesity in children (Fig. 2).
Metabolic features in obesity and their relationship with vitamin D supply
When analyzing metabolic comorbidity in children and adolescents with obesity, the most significant disorders were represented by insulin resistance in more than half of those examined, as well as hypercholesterolemia and hypertriglyceridemia, respectively, in 23% for each position, and dyslipidemia, represented by a decrease in HDL, in 41% of those examined. In the more severely obese group, the median fasting insulin level was higher compared to children with moderate obesity, and the number of children with the above-mentioned disorders was comparable in both groups, regardless of the severity of obesity (Table 2).
When assessing the supply of vitamin D in a group of school-age children with obesity in a comparative aspect with children of normal weight matched by age and gender, results were obtained indicating a comparable low level of provision in both groups, regardless of body weight. Thus, the medians were 16.8 ng/ml and 17.8 ng/ml, respectively; the differences were not statistically significant (p > 0.05). Only 8% of children in the obese group and 11% in the control group had a normal supply of vitamin D, while the majority in both groups corresponded to a deficiency of this vitamin (Fig. 3).
When comparing vitamin D levels depending on the severity of obesity and gender, no significant differences were also found. However, it was of interest that in an in-depth analysis of children who had vitamin D deficiency, the median 25(OH)D in the obese group was statistically significantly lower compared to that in the non-obese group (14.1 ng/ml vs. 16.4 ng/ml, p = 0.0005), indicating that in the formally same group classified as vitamin D deficiency, the degree of deficiency was more pronounced in the presence of obesity.
When studying the vitamin D supply within the obese group, depending on the stage of puberty, data were obtained on significantly lower values of 25(OH)D in adolescents who had entered puberty compared to pre-pubertal children (medians, respectively, 15.8 ng/ml and 21.1 ng/ml, p = 0.006), and the lowest 25(OH)D values were detected precisely in the vitamin D deficiency group (medians, respectively, 13.1 ng/ml and 16.1 ng/ml, p = 0.02) ( Table 3). In the group of children without obesity, the presence of puberty did not have a similar effect on the level of vitamin D provision.
Thus, a low supply of vitamin D has been established among school-age children and adolescents, regardless of body weight, severity of obesity and gender. However, the presence of obesity and puberty determined a lower level of 25(OH)D within the group of its deficiency.
Both obesity and vitamin D deficiency are known to have negative effects on carbohydrate and lipid metabolism. When studying metabolic status disorders, it was found that insulin resistance and decreased HDL levels were more common among children with obesity and vitamin D deficiency. Medians of the HOMA-IR index (3.95 versus 2.65, p = 0.0099), fasting insulinemia (127, 05 pmol/l versus 82.15 pmol/l, p = 0.0089), glycemia at the 120th minute of the glucose tolerance test (6.12 mmol/l versus 5.15 mmol/l, p = 0.02), triglycerides (1.11 mmol/l vs. 0.95 mmol/l, p = 0.045) were higher in this group compared to the group of obese children with normal vitamin D status (Table 4).
When analyzing the contribution of puberty age to the studied disorders, a higher peak glucose level was also established during the oral glucose tolerance test in the group of adolescents with obesity and vitamin D deficiency compared to obesity and normal nutrition (6.12 mmol/l versus 5.37 mmol/l). l, p = 0.029), while comparison of this parameter in comparable groups of children with no onset of puberty revealed no differences (p = 0.59).
When studying the relationship between changes in metabolic status and the levels of leptin and adiponectin in children with obesity and different levels of vitamin D, it was found that in obesity, the level of leptin increased severalfold compared with the corresponding data in children without excess adipose tissue (51.6 ng/ l and 4.1 ng/ml, p = 0.001). Moreover, within the group of obese children, leptin levels increased statistically significantly with more severe degrees of this pathology (46.95 ng/ml and 76.47 ng/ml, respectively, p = 0.001). With regard to adiponectin, no similar results were obtained in these groups (Table 5).
Correlation analysis revealed significant direct connections between leptin levels and BMI and IMI, as well as with the growth of both total fat and differentiated fat in android and gynoid localizations, and the most pronounced relationships were established in the group of children with SDS BMI ≥ +3 (Fig. 4 ).
Results were obtained indicating a significantly lower leptin level with normal 25(OH)D levels compared to the group with reduced levels (52.84 ng/ml and 26.3 ng/ml, respectively, p = 0.048) (Table 6).
The study of the dynamics of changes in adiponectin did not reveal significant differences in groups of obese children in general compared with controls with normal weight, as well as in groups depending on the severity of obesity and the degree of vitamin D provision. Correlation analysis found that the level of adiponectin in the group of prepubertal children age had statistically significant direct correlations with the level of HDL and inverse correlations with the level of fasting glycemia and triglyceride levels (Fig. 5).
Conclusion
Based on the totality of the results obtained during the study, a “clinical portrait” of a child with obesity was compiled and the most severe metabolic and cardiovascular disorders were predicted: a teenager with the onset of puberty, with severe obesity, with a BMI > +3 SDS, with a vitamin D deficiency (25(OH)D < 20 ng/ml) (Fig. 6).
From the point of view of providing assistance and preventing adverse events, assessing the “controllability” of these factors, it should be recognized that the last one is the most controllable, which justifies the importance of timely and adequate correction of vitamin D supply.
To provide the child's body with vitamin D, cholecalciferol preparations (vitamin D3) are widely used, which are presented on the Russian pharmaceutical market in various forms (aqueous and oily solution, capsules, tablets) and dosages. Detrimax® Baby is a pure oil solution (medium chain triglycerides) and contains 200 IU of cholecalciferol in 1 drop. Detrimax® Baby has a unique pump dispenser that allows you to accurately and quickly measure the required dose of vitamin D. When dosing, there is no need to invert the bottle of the drug, which reduces the possibility of spilling. The pump dispenser makes it impossible to accidentally increase the dose, which is a problem with other drugs that have a standard plastic dropper.
Literature
- Karonova T. L., Tsvetkova E. V., Klyushina A. A., Mikheeva E. P., Belyaeva O. D., Kostareva A. A., Grineva E. N. Level of 25(OH) vitamin D and components metabolic syndrome in women of reproductive age with different genotypes of the ApaI polymorphism of the vitamin D receptor gene // Arterial hypertension. 2013, vol. 19, no. 1: 67–75.
- Vimaleswaran KS, Berry DJ, Lu C., Tikkanen E., Pilz S. et al. Causal relationship between obesity and vitamin D status: bi-directional Mendelian randomization analysis of multiple cohorts // PLoS Med. 2013; 10 (2).
- Liu E., Meigs JB, Pittas AG et al. Predicted 25-hydroxyvitamin D score and incident type 2 diabetes in the Framingham Offspring Study // The American journal of clinical nutrition. 2010; 91(6); 1627–1633.
- Gagnon C., Lu ZX, Magliano DJ, Dunstan D. et al. Serum 25-hydroxyvitamin D, calcium intake, and risk of type 2 diabetes after 5 years // Diabetes care. 2011; 34(5); 1133–1138. DOI: 10.2337/dc10–2167.
- Pittas AG, Sun Q., Manson JE, Dawson-Hughes B. et al. Plasma 25-hydroxyvitamin D concentration and risk of incident type 2 diabetes in women // Diabetes care. 2010; 33(9); 2021–2023. DOI: 10.2337/dc10–0790.
- George PS, Pearson ER, Witham MD Effect of vitamin D supplementation on glycemic control and insulin resistance: a systematic review and meta-analysis // Diabetic Medicine. 2012; 29(8); e142–150. Doi: 10.1111/j.1464–5491.2012.03672.x.
- Zakharova I.N. et al. Vitamin D deficiency in adolescents: results of year-round screening in Moscow // Pediatric Pharmacology. 2015. No. 12 (5). pp. 528–531.
- Borisenko E.P. Vitamin D supply of children and adults in the Amur region // Bulletin of pathology and physiology of respiration. 2021. No. 60. pp. 57–61.
- Klimov L. Ya., Pludovsky P., Zakharova I. N. Modern views on enriching the diet of children and adults with vitamin D: problems and prospects // Consilium Medicum. Pediatrics. 2021. No. 3. pp. 10–17.
- Maltsev S.V. Vitamin D provision in children of different age groups in winter // Russian Bulletin of Perinatology and Pediatrics. 2021. No. 62 (2). pp. 99–103.
- Zakharova I. N. et al. Vitamin D deficiency in young children in Russia: results of the multicenter cohort study Fontanel (2013–2014) // Issues of modern pediatrics. 2014. No. 13 (6). pp. 30–34.
- Zakharova I.N. et al. Vitamin D deficiency in adolescents: results of year-round screening in Moscow // Pediatric Pharmacology. 2015. No. 12 (5). pp. 528–531.
- Rusconi RE et al. Vitamin D insufficiency in obese children and relation with lipid profile // International Journal of Food Sciences and Nutrition. 2015. Vol. 66, No. 2. P. 132–134.
- Moschonis G. et al. Vitamin D insufficiency is associated with insulin resistance independently of obesity in primary schoolchildren. The healthy growth study // Pediatric Diabetes. 2018. Vol. 19, No. 5. P. 866–873.
- Holick MF et al. Evaluation, Treatment, and Prevention of Vitamin D Deficiency: an Endocrine Society Clinical Practice Guideline // The Journal of Clinical Endocrinology & Metabolism. 2011. Vol. 96, No. 7. P. 1911–1930.
- P?udowski P. et al. Practical guidelines for the supplementation of vitamin D and the treatment of deficits in Central Europe - recommended vitamin D intakes in the general population and groups at risk of vitamin D deficiency // Endokrynologia Polska. 2013. Vol. 64, No. 4. P. 319–327.
- Pludowski P. et al. Vitamin D supplementation guidelines // The Journal of steroid biochemistry and molecular biology. 2021. Vol. 175. P. 125–135.
- Zazerskaya I. E. et al. Vitamin D and women’s reproductive health. St. Petersburg: Eco-Vector LLC, 2021. 151 p.
- Karonova T.L. Shmonina I.A., Totolyan N.A. Multiple sclerosis and the level of vitamin D supply // Arterial hypertension. 2015. No. 21 (2). pp. 121–129.
- Naumov A.V. Hormone D3 as a vitamin for comorbid conditions: to whom, when and how? // Difficult patient. 2021. No. 16 (3). pp. 20–27.
- Holick MF The D-Lightful Vitamin D for Child Health // Journal of Parenteral and Enteral Nutrition. 2012. Vol. 36, No. 1. P. 9–19.
- Kumar J. et al. Prevalence and Associations of 25-Hydroxyvitamin D Deficiency in US Children: NHANES2001–2004 // Pediatrics. 2009. Vol. 124, No. 3. P. 362–370.
- Torun E. et al. The clinical and biochemical presentation of vitamin D deficiency and insufficiency in children and adolescents // Journal of Pediatric Endocrinology and Metabolism. 2013. Vol. 26, no. 5–6. P. 469–475
- Pigarova E. A., Rozhinskaya L. Ya., Belaya Zh. E. et al. Clinical recommendations of the Russian Association of Endocrinologists for the diagnosis, treatment and prevention of vitamin D deficiency in adults // Probl. endocr. 2021. T. 62. No. 4. pp. 60–84.
- Karonova T., Andreeva A., Nikitina I., Belyaeva O., Mokhova E., Galkina O., Vasilyeva E., Grineva E. Prevalence of Vitamin D deficiency in the North-West region of Russia: A cross-sectional study // The Journal of steroid biochemistry and molecular biology. 2016; 164:230–234. DOI: 10.1016/j.jsbmb.2016.03.026.
- Alamdari A., Mozafari R., Tafakhori A. et al. An inverse association between serum vitamin D levels with the presence and severity of impaired nerve conduction velocity and large fiber peripheral neuropathy in diabetic subjects // Neurological Sciences. 2015; 36(7):1121–1126. DOI: 10.1007/s10072–015–2207–0.
- Nikitina I. L., Karonova T. L., Grineva E. N. Vitamin D deficiency and health // Arterial hypertension. 2010. T. 16., No. 3. P. 277–281.
- Demerath EW, Johnson W. Pediatric body composition references: what's missing? // The American journal of clinical nutrition. 2013. Vol. 98, No. 1. P. 1–3.
I. L. Nikitina1, Doctor of Medical Sciences A. M. Todieva, Candidate of Medical Sciences
FSBI NMC named after. V. A. Almazova Ministry of Health of Russia, St. Petersburg
1 Contact information
DOI: 10.26295/OS.2019.60.22.005
Vitamin D deficiency in obese children - who is at highest risk? / I. L. Nikitina, A. M. Todieva For citation: Attending physician No. 12/2019; Issue page numbers: 31-37 Tags: metabolic syndrome, cardiovascular disorders, cholesterol