Saxenda - reviews
Review:
A little background... I am 29 years old, height 164, weight a week ago was 102.5 kg, clothing size 54-56-58 (large breasts)... I have insulin resistance since the days of walking under the table. Throughout my childhood, I spent my entire childhood running around to doctors and heard only one thing: “DIETS DIETS DIETS!!!!!”, until one day, at the age of 19, I saw an intelligent doctor, who diagnosed this disease + PCOS (at that time I weighed about 90 kg) . With grief in half, in 3 years my body, as they say, was put back on its feet. Through treatment with hormones and Metformin, I reached the 60 kg mark. from that moment on, I completely gave up sugar, was always active and ate healthy, with the exception of “fasting” days (major holidays). But unfortunately the positive picture did not last long. 2 - 2.5 years after serious stress, my body sent me to hell and everything began to return to normal. (despite the fact that I still stuck to 1200-1500 kcal and without sugar and flour). It turns out that by the age of 26 I again returned to the starting point. The weight grew wildly, and in a month I could gain from 2 to 5 kg. I decided to see a doctor again, but unfortunately the doctor who saw me retired. Contact with her was lost. I grieved... Then there was a period of “money down the drain.” I changed several doctors and had a lot of tests. There is only one answer - DIET!!!! I began to realize that depression was approaching. I decided to independently repeat the previously completed course of treatment. Effect - 1 kg per month.
Some time later I gave up everything. My husband demanded that I stop with self-criticism and finally give myself a mental break. (thanks to him for that). And now, half a year later, after studying a bunch of reviews and recommendations from friends, I decided to go to the doctor again. Which I didn’t regret. She is the only one who was interested in “why is this so?”, who cared that I was suffering. After sitting at the reception for about an hour, the doctor studied absolutely the entire history of my illness, I gave her everything that I could have left in my hands that I could save from research, and we jointly made a decision - we need to give a push. you can't hesitate. Especially if I want to become a mother in the future. And so, the doctor decided that it would be advisable to start using Saxenda. I’ll be honest, it took me a long time to decide whether to take it or not (expensive, side effects, consequences, all these thoughts haunted me). The doctor assured that it is prescribed even to children from 12 years of age, but strictly under supervision and individual dosage. But this is a very serious drug. Moreover, he is relatively “young” in medicine. I struggled and struggled, but finally decided. The doctor prescribed injections in combination with taking Glucophage Long 1000. Exactly a week has passed. Injection at a dosage of 0.6 + half of Glucophage, all before bed. What a week I managed to notice. There is less swelling in the legs, 4 kg are gone. weight, periodic “cloudiness” with nausea occurs. Fresh air + mint chewing gum helps you quickly get back into the flow. Suppresses appetite. My diet: morning - coffee, cheese sandwich; lunch - broth or cottage cheese; dinner - steamed meat (chicken breast, beef), a couple of fresh cucumbers. I stuff all this into myself. I'm worried that my stomach won't thank me. and of course some water. I try to drink constantly, and in fact I’m almost always thirsty))))) I haven’t measured how much I drink. But more than usual.
I would also like to add something of my own. GIRLS!!!! Saxenda is a medicinal product, not a dietary supplement. It should appear in your first aid kit only (!) as prescribed by a doctor. If you weigh 55 - 65 kg and want a wonderful effect from this drug, think three hundred times whether it will harm you?!
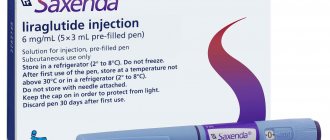
Saxenda solution for subcutaneous injection filled syringe pens 6 mg/ml 3 ml N 5
Active ingredients
Liraglutide
Compound
1 ml of the drug contains: active ingredient: liraglutide 6 mg (one pre-filled syringe pen contains 3 ml of solution, which corresponds to 18 mg of liraglutide); excipients: sodium hydrogen phosphate dihydrate 1.42 mg, phenol 5.5 mg, propylene glycol 14.0 mg; hydrochloric acid/sodium hydroxide (for pH correction), water for injection up to 1 ml.
Mechanism of action
The active substance of Saxenda®, liraglutide, is an analogue of human glucagon-like peptide-1 (GLP-1), produced by recombinant DNA biotechnology using the Saccharomyces cerevisiae strain, which has 97% amino acid sequence homology to endogenous human GLP-1.
Liraglutide binds to and activates the GLP-1 receptor (GLP-1R). Liraglutide is resistant to metabolic degradation, its half-life from plasma after subcutaneous administration is 13 hours. The pharmacokinetic profile of liraglutide, which allows it to be administered to patients once a day, is the result of self-association, which results in delayed absorption of the drug; binding to plasma proteins; and resistance to dipeptidyl peptidase-4 (DPP-4) and neutral endopeptidase (NEP). GLP-1 is a physiological regulator of appetite and food consumption. GLP-1R is found in several brain regions involved in appetite regulation. In animal studies, administration of liraglutide resulted in its uptake into specific areas of the brain, including the hypothalamus, where liraglutide, through specific activation of GLP-1R, increased satiety signals and decreased hunger signals, thereby leading to weight loss.
Liraglutide reduces body weight in humans primarily by reducing adipose tissue mass. Reducing body weight occurs by reducing food consumption. Liraglutide does not increase 24-hour energy expenditure. Liraglutide regulates appetite by increasing feelings of fullness and satiety, while reducing feelings of hunger and reducing expected food intake. Liraglutide stimulates insulin secretion and reduces excessive glucagon secretion in a glucose-dependent manner, and also improves pancreatic beta cell function, which leads to a decrease in fasting and postprandial glucose concentrations. The mechanism for lowering glucose concentrations also involves a slight delay in gastric emptying.
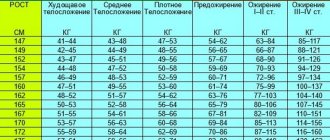
Indications Saxenda® is indicated as an adjunct to a low-calorie diet and increased physical activity for long-term use to correct body weight in adult patients with a BMI: - ≥30 kg/m2 (obesity) or - ≥ 27 kg/m2 to < 30 kg/ m (overweight) in the presence of at least one comorbidity associated with excess body weight, such as impaired glucose tolerance, type 2 diabetes mellitus, arterial hypertension, dyslipidemia or obstructive sleep apnea syndrome.
Contraindications
- hypersensitivity to liraglutide or any of the auxiliary components of the drug;
- history of medullary thyroid cancer, including family history;
- multiple endocrine neoplasia type 2;
- severe depression, suicidal thoughts or behavior, including a history.
Use is contraindicated in the following groups of patients and for the following conditions/diseases due to the lack of data on efficacy and safety:
- severe renal dysfunction;
- severe liver dysfunction;
- children under 18 years of age;
- period of pregnancy and breastfeeding;
- heart failure of functional class III-IV (according to the NYHA (New York Heart Association) classification);
- simultaneous use of other drugs for weight correction;
- simultaneous use with insulin;
- secondary obesity due to endocrinological diseases or eating disorders, or due to the use of medications that can lead to weight gain.
Before use, consultation with a specialist is required. The method of use and dosage regimen of a particular drug depend on its release form and other factors. The optimal dosage regimen is determined by the doctor. Storage regimen, interactions and side effects are indicated in the instructions.
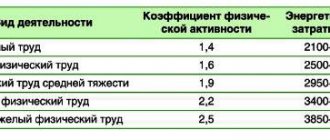
The steady increase in the number of patients with type 2 diabetes mellitus (T2DM), especially significant in recent years, forces us to constantly revise forecasts [1]. Many of the causes of this epidemic are now well understood. It has been shown that population aging leads to a natural increase in the incidence of T2DM. Studies suggest that there are age-related mechanisms for increasing plasma glucose levels, especially after meals. It is believed that every 10 years after the age of 50, the fasting glucose level increases slightly, and the postprandial concentration increases by 0.5 mmol/l [2]. In this regard, population aging can be considered an uncorrectable risk factor for diabetes, especially in economically developed countries. However, excess body weight has a much greater share in the pathogenesis of T2DM. Obesity is common among both elderly and middle-aged people, constituting 40% of the population [3]. It is obesity, which usually develops after 35-40 years and is called android, viscero-abdominal obesity, that is the basis for the development of metabolic syndrome - insulin resistance syndrome [4]. However, a decrease in tissue sensitivity to insulin is not the only factor leading to the development of T2DM. Without disruption of the secretory ability of β-cells and a decrease in their number, T2DM does not manifest itself. Chronologically, the first link in the development of T2DM is the insulin resistance characteristic of patients with viscero-abdominal obesity. In the case of preserved secretory ability of β-cells, excess insulin secretion compensates for insulin resistance for some time and allows maintaining normoglycemia for a fairly long period (up to 10 years). Over time, there is a gradual depletion of the secretory ability of β-cells, as well as a significant decrease in their number. Hyperglycemia and dyslipidemia, characteristic of this period of the disease, aggravate β-cell dysfunction, significantly complicating the treatment of such patients. It must be taken into account that, due to long-term previous hyperinsulinemia, most obese patients by the time diabetes is diagnosed already have a number of cardiovascular diseases, in particular, arterial hypertension and various clinical manifestations of atherosclerosis of the coronary, cerebral and peripheral arteries. Therefore, to slow the progression of these diseases with the development of diabetes, hyperglycemia should be eliminated as quickly as possible.
At the same time, intensive glycemic control is associated with the risk of hypoglycemic conditions, which are extremely dangerous in patients with high cardiovascular risk. Considering the fact that more than half of patients with T2DM have cardiovascular diseases or multiple risk factors for them, the algorithms for the treatment of patients with T2DM developed by the Russian Association of Endocrinologists (2011) as first-line drugs, both for starting and for intensifying therapy, recommend drugs , which have minimal ability to induce hypoglycemia [5].
These drugs include biguanide derivatives, dipeptidyl peptidase-4 (DPP-4) inhibitors, and glucagon-like peptide-1 (GLP-1) agonists. While biguanide derivatives are well-tested drugs, DPP-4 inhibitors and GLP-1 agonists are a new class of incretin drugs and there is little experience with their use in clinical practice. These drugs have the unique effect of incretins, which regulate glucose homeostasis in a glucose-dependent manner [6], positively influencing all stages of insulin biosynthesis and secretion, as well as preventing depletion of the insulin pool in secretory granules. Endogenous incretin, GLP-1, provides up to 70% of postprandial insulin secretion by β-cells, while simultaneously suppressing glucagon production by α-cells of the islets of Langerhans [7]. When the blood glucose level decreases to 4.5 mmol/l, the stimulating effect of GLP-1 on the insulin response ceases, and with hypoglycemia, GLP-1 begins to stimulate the activity of pancreatic α-cells. As a result, glucagon levels increase and the physiological production of glucose by the liver increases, which helps restore normal glycemic levels. Thus, the risk of developing hypoglycemia during incretin therapy is practically absent. In addition, as shown in experimental studies, GLP-1 stimulates the proliferation of β-cells and suppresses their apoptosis [8]. Incretins also have additional glucose-lowering effects by promoting the utilization of glucose by fat and muscle cells and suppressing glucose production by the liver. In other words, they reduce insulin resistance.
Incretin therapy does not cause weight gain and does not have a negative effect on blood pressure (BP) or blood lipid levels [9]. According to the recommended treatment algorithms for patients with T2DM (2011), GLP-1 agonists, which effectively reduce body weight and systolic blood pressure, are preferred as first-line drugs at the onset of the disease in the presence of obesity and arterial hypertension.
In recent years, a number of pleiotropic effects of incretins mediated by G proteins have been identified. GLP-1 receptors are found not only in the pancreas, but also in the cells of the intestines, stomach, kidneys, heart, lungs, as well as in the cells of the peripheral and central nervous system. Stimulation of GLP-1 receptors in the ileum leads to increased vagal effect, decreased gastrointestinal motility, and slower gastric emptying. This effect probably explains faster satiety and contributes to decreased appetite [10]. GLP-1 receptors are also found in the nuclei of the hypothalamus, which regulate eating behavior, the stimulation of which also leads to a decrease in appetite and body weight [11]. Administration of GLP-1 agonists to animals with heart failure increases cardiac output, and in the presence of myocardial ischemia, reduces the infarct area [12].
Extremely important is data on the existence of a GLP-1 metabolite that can stimulate NO-dependent vasodilation of the coronary arteries, which suggests that GLP-1 has a cardioprotective effect [13].
The effect of GLP-1 on the kidneys is associated with increased natriuresis and diuresis, presumably due to its direct effect on Na+/H+ transport in the proximal tubules of the kidneys. In addition, incretins, without having a direct effect on glomerular filtration in healthy people, reduce hyperfiltration in insulin-resistant patients with increased body weight [14]. There are experimental data on the effect of GLP-1 on bone tissue. It is assumed that their protective effect appears to occur through calciotonin-dependent mechanisms.
In patients with T2DM and even in those with prediabetes, incretin effects are reduced, which impairs the release of insulin in response to carbohydrate intake [15]. To correct these disorders, it has been proposed to use two groups of drugs: DPP-4 inhibitors and GLP-1 analogues. The use of drugs that block the DPP-4 enzyme (sitagliptin, vildagliptin, saxagliptin) leads to the maintenance of physiological levels of GLP-1 and an increase in the half-life of endogenous GLP-1. However, in patients with T2DM, the sensitivity to GLP-1 is significantly reduced and to restore its effects, a concentration of GLP-1 is required that is several times higher than physiological, which explains the lack of a significant hypoglycemic effect when treated with DPP-4 inhibitors in a number of patients. At the same time, the use of GLP-1 agonists that are not affected by DPP-4 seems very promising.
One of these drugs is a long-acting analogue of human GLP-1 - liraglutide (Victoza), registered in Russia in May 2010. Unlike other drugs of this series used today in clinical practice, Victoza has a high affinity for endogenous human GLP-1 receptors. 1 and is resistant to proteolytic degradation by DPP-4. The half-life of the drug reaches 13 hours, and the duration of action is up to 24 hours, which allows the drug to be administered only once a day [16].
This paper presents the experience of using the drug Victoza in the treatment of patients with T2DM.
The drug was prescribed both in a hospital setting and on an outpatient basis. The main indications for prescribing Victoza were: confirmed type 2 diabetes, lack of glycemic control while taking average or maximum therapeutic doses of one or two oral glucose-lowering drugs (OALDs), as well as the presence of obesity or overweight. A prerequisite for prescribing Victoza was the preservation of the functional activity of β-cells, confirmed by normal or increased levels of C-peptide. Most patients had concomitant pathology of the cardiovascular system, arterial hypertension and atherogenic dyslipidemia. In this regard, almost all observed patients received antihypertensive, lipid-lowering drugs and antiplatelet agents, so it was very difficult to assess the additional pleiotropic effects of the GLP-1 analogue. Polypharmacy, characteristic of the treatment of patients with T2DM, is usually caused by the desire to achieve not only glycemic control, but also target blood pressure levels, blood lipid profile, as well as the need to treat concomitant diseases (Table 1).
However, during the initial examination before prescribing Victoza, in almost all patients, most control indicators exceeded target values. The observation group consisted of patients with an initial level of glycated hemoglobin (HbA1c) above 8%.
When prescribing Victoza, the instructions for using this drug were strictly followed. Thus, none of the patients received insulin, and irrational combinations of PSSPs (for example, DPP-4 inhibitors and GLP-1 agonist) were not used. Among the patients there were no persons suffering from cancer, organic diseases of the central nervous system, severe cardiac, pulmonary, renal or liver failure.
The drug Victoza was prescribed subcutaneously at an initial dose of 0.6 mg once a day at the same time, regardless of meals, with a subsequent dose increase to 1.2 mg/day.
Data from 6-month treatment of 71 patients with T2DM are presented, among whom there were 45 men and 26 women; the average age was 51.1±5.2 years with a disease duration of 6.4±4.2 years. In most cases, these were patients with severe obesity (average BMI was 35.8±2.3 kg/m2). All patients had poor glycemic control. The HbA1c level averaged 8.9±1.2%. Before starting therapy with Victoza, 14 patients received monotherapy with sulfonylureas (SMU), 17 received monotherapy with metformin, 23 received combination therapy with two SUDs (PSM + metformin), and 19 did not receive drug therapy for diabetes.
In the analyzed group, all glycemic indicators were quite high: fasting plasma glucose - 9.8 ± 0.8 mmol/l, postprandial glycemia - 12.1 ± 1.8 mmol/l. The majority of patients had arterial hypertension (42 people, 59%), all examined had atherogenic dyslipidemia.
The results of treatment with Victoza were assessed 3 and 6 months after the start of its use (Table 2).
After just 3 months, the HbA1c level almost reached the target level. After 6 months of treatment with Victoza, the HbA1c level returned to normal and amounted to 6.6%. Fasting plasma glucose concentrations and postprandial glucose concentrations at 6 months also reached target values. Moreover, not a single case of severe hypoglycemia was noted in any patient within 6 months. Mild hypoglycemic conditions were observed rarely in patients receiving Victoza in combination with other PSSPs: in 8 out of 31 patients receiving Victoza in combination with PSM. In patients who initially did not receive glucose-lowering drugs, the HbA1c level reached target values after 3 months of treatment and amounted to 6.9±1.1%, and after 6 months - 6.5±0.9%.
The presented data confirm that the drug Victoza has a pronounced hypoglycemic ability, comparable to that of the most active non-insulin drugs used in the treatment of T2DM. After six months of using the drug Victoza in patients with T2DM and poor glycemic control, including against the background of previous PSSP therapy, it was possible to achieve ideal control in the absence of severe hypoglycemic conditions.
It should be emphasized that the administration of the drug Victoza led not only to a significant improvement in glycemic control in all patients presented; in patients who had previously received PSSP therapy and did not achieve optimal control, the addition of Victoza made it possible to reduce the dose of PSSP (in 11 of 14 patients on PSM therapy and in 11 of 23 patients who had previously received PSM in combination with metformin) or completely cancel secretagogues (in 3 patients who had previously received PSM, and in 9 patients who had previously received PSM + metformin).
It is extremely important to reduce body weight in all patients receiving the drug Victoza. The weight loss ranged from 1.3 to 16.5 kg. On average, 3 months after the start of treatment, body weight decreased by 5.1 kg, and after 6 months - by 6.4 kg. The greatest reduction in body weight was observed in patients with an initially more severe degree of obesity. Thus, in the group of patients with a BMI of 30-35 kg/m2, the decrease in body weight after 3 months was 3.3 kg, and after 6 months - 3.9 kg. In the group of patients with a BMI of 35-40 kg/m2, body weight decreased by 4.6 and 5.9 kg after 3 and 6 months, respectively. Patients with morbid obesity (BMI above 40 kg/m2) experienced the most pronounced weight loss: by 7.6 kg after 3 months and by 10.1 kg over six months. It was not possible to identify any relationship between the dynamics of the decrease in glycemic levels and HbA1c and BMI. It should be noted that all patients followed a moderately restrictive diet (1700-1800 kcal per day), but during treatment with Victoza they were able to follow nutritional recommendations much more easily than before. However, the dynamics of body weight did not depend on the presence and severity of dyspeptic symptoms.
In addition to the listed clinical effects, therapy with Victoza in patients with T2DM was accompanied by a significant decrease in serum cholesterol and triglyceride levels. Total cholesterol decreased over 6 months from 6.7 to 5.1 mmol/l, and triglyceride levels from 2.3 to 1.5 mmol/l. It should be noted that the majority of patients received certain lipid-lowering drugs (statins or fibrates) according to indications, but their doses were stable throughout the observation period. A decrease in the severity of dyslipidemia can be explained, on the one hand, by improved glycemic control and a decrease in body weight, and on the other, by a decrease in the dosage of PSM.
Of the 71 patients with T2DM, 52 received chronic antihypertensive therapy, including ACE inhibitors or angiotensin II receptor blockers, and 29 patients were additionally prescribed calcium channel blockers. Correction of antihypertensive therapy was carried out before patients were included in the study. During observation, none of the patients experienced hypertensive crises or significant increases in blood pressure. During the entire observation, the majority of patients managed to maintain target blood pressure values; This was partly due to the fairly high compliance of patients. At the same time, the own hypotensive effect of the drug Victoza cannot be excluded, since the natriuretic effect of incretins is known. In 9 patients, it was necessary to reduce the dose of antihypertensive drugs due to a tendency to hypotension, which may have been associated with a decrease in body weight.
Therapy with Victoza was well tolerated. None of the patients complained of nausea or other dyspeptic disorders. The most common sensation when starting to use the drug Victoza was a feeling of being full more quickly than before with the usual amount of food. At the same time, appetite and pleasure from eating were preserved. Two patients noted a tendency to constipation at the beginning of therapy; regular bowel movements were restored over the next 3-4 days. The use of laxatives was not required in any case.
Thus, the study showed that in patients with T2DM, Victoza is a very effective and safe drug both for achieving good glycemic control and in terms of diabetes control in general. The undeniable additional advantages of therapy with Victoza are the ability to effectively reduce excess body weight, improve the blood pressure profile and blood lipid levels. The data obtained allow us to recommend wider use of the drug Victoza, both as monotherapy in the early stages of the disease and in the complex treatment of T2DM.
How we are treated: Saxenda
But scientists doubt the ability to prevent type 2 diabetes with such drugs. The effect of liraglutide on the risk of developing non-insulin-dependent diabetes has not yet been proven, despite a number of studies in which it reduces the blood levels of certain substances that are precursors of the disease. The long-term effects of the drug on blood pressure in obese patients are also largely unknown.
Indicator.Ru recommends: take under medical supervision
The drug is available by prescription, so self-medication is unlikely to be a problem. And rightly so: with an increased dosage, you can reduce blood sugar levels to dangerous levels (this is called hypoglycemia), resulting in fainting or worse as a reward. And no one has canceled the risks due to incorrect injections. Most patients are unlikely to face anything serious: the most common side effects, which occur in about 5% of patients, will not be dangerous, but rather unpleasant, such as nausea or constipation.
The drug is recommended for patients with a body mass index above 30 (or above 27, but with complications). Taking it for the sake of wanting to lose a couple of kilos from your 60, and even without type 2 diabetes, will be dangerous, but, for example, if your body weight is 90 kg with a height of 165 cm, such indicators can already be considered justified. The medicine will really be effective and will help you lose about 6% of your body weight, which is more than a number of other weight loss drugs.
There are suspicions that the drug increases the risk of thyroid and pancreatic cancer, as well as pancreatitis and heart problems, but they have so far only been indirectly confirmed. If such a danger exists, it occurs infrequently.
But who should not use Saxenda are patients with impaired kidney and liver function, pancreatitis, gallbladder diseases, and also (the effect is not fully proven, but it is better to be careful) people with suicidal tendencies. It is not recommended for patients with heart palpitations at rest. During pregnancy and breastfeeding, safety for the child has not been proven, so it is better not to take risks.
The combination of the drug with other diabetes medications should be very thoughtful: it can greatly enhance the effect of some, while it will interfere with others, so consult your doctor. Warfarin and other coumarin derivatives should be used with caution.
In addition, do not forget about a healthy lifestyle: the drug can be a good addition to a diet with a calorie deficit and physical activity, but without them, the results are unlikely to impress you.
Our recommendations cannot be equated with a doctor's prescription. Before you start taking this or that drug, be sure to consult a specialist.
Did you like the material? Add Indicator.Ru to “My Sources” of Yandex.News and read us more often.
Subscribe to Indicator.Ru on social networks: Facebook, VKontakte, Twitter, Telegram, Odnoklassniki.