Main
Saxenda (liraglutide) is approved by the US Food and Drug Administration (FDA) for use in adolescents (12 years of age and older) as an adjunct to a low-calorie diet and vigorous exercise for weight management. The drug is approved if the weight exceeds 60 kg and the body mass index (BMI) adjusted for the age and sex of adolescents is ≥ 30 kg/m2, which corresponds to obesity in adults.
Previously, Saxenda, introduced by Novo Nordisk in 2014, was approved for use only by adults.
Connecting adolescent patients to Saxenda is an important event. First, over the past 20 years, the prevalence of overweight among children and adolescents has doubled: from 10% to 20%. It has been proven that if both parents have problematic weight, then in 80% of cases the same will be true for the child. In general, excess weight is a global problem: in 2021, there were more than 1.9 billion adults (including over 650 thousand obese) who could use weight loss in one way or another.
Second, orlistat and phentermine are the only drugs approved by the FDA for use in the pediatric population (ages 12 and 16 years, respectively). There are no approved drugs against obesity in children and adolescents in Europe. In addition, bariatric surgery, as the most effective way to remove extra pounds, is only offered to teenagers if they are severely obese.
"Saxenda" (Saxenda, liraglutide).
Dosage and overdose
The initial dose for an adult usually contains a maximum of 0.6 mg per day, the maximum should be prescribed - 3 mg per day. One syringe contains 3 ml, which contains 6 mg of the main substance.
Important! How long does the package last, that is, 9 ml of the drug. One dose – 0.6 mg – is 0.3 ml, one syringe is enough for 10 doses, and three – for 30, for 1 month of use.
The maximum therapeutic effect should occur after 12 weeks of the course; if body weight has not decreased by more than 5% during this time at the maximum permissible dose of 3 mg per day, therapy should be discontinued.
Elderly people over 65 years of age do not require dose adjustment, as do people with chronic diseases of the urinary and biliary systems with mild or moderate renal or liver failure. In severe forms, it is worth consulting with specialists, a hepatologist and a nephrologist to decide on the prescription of the drug.
Each of these special syringe pens is very easy to use. It has scales showing how much to inject and how much medication is left. Each package contains detailed instructions on how to properly use the pen and how to properly store it once opened.
Cases of overdose have been described, the maximum dose was 72 mg. At this concentration, the drug can cause harm to the body. Overdose symptoms:
- Hypoglycemia, but without establishing hypoglycemic shock;
- Severe nausea and frequent vomiting with abdominal pain.
All cases are treated symptomatically with infusion therapy.
What is dyslipidemia
Details
The phase III clinical trial NCT02918279 (randomized, double-blind, placebo-controlled, multicenter, international) examined the safety and effectiveness of weight management among obese adolescent patients (n=251) (12–17 years).
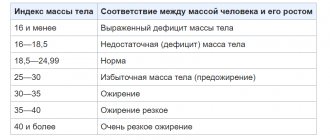
Obesity was defined as a BMI of 30 kg/m2 or more for adults, which, due to heterogeneity, was adjusted according to international criteria for adolescent BMI and at least the 95th percentile of an age- and sex-matched reference value.
Participants were prescribed placebo or liraglutide at a maximum dose of 3.0 mg (the dose was increased gradually to the maximum tolerated dose) by daily subcutaneous injections for 56 weeks. The subjects had to adhere to a course of lifestyle changes.
The primary endpoint was the change in BMI using standard deviation (SDS) scores. The latter, also known as the Z-score, was the number of standard deviations from the population average BMI adjusted for age and sex.
The liraglutide group outperformed the control group in all respects:
The average absolute change in BMI was −1.6 ±3.1 kg/m2 versus +0.1 ±3.4 kg/m2. The average absolute change in body weight came out to −2.7 ±9.1 kg - versus +1.7 ±10.1 kg.
After discontinuation of liraglutide, weight indicators began to increase again, and in the Saxenda group to a greater extent than in the control group: the estimated difference between groups, according to BMI according to SDS, was 0.15 (95% CI: 0.07–0.23 ). However, after 82 weeks, the therapeutic effect still persisted: the average absolute change in BMI was −0.2 ± 3.5 kg/m2 - versus +0.8 ± 4.0 kg/m2, the average absolute change in body weight was + 1.7 ±10.1 kg - versus +4.4 ±11.7 kg.
Among the most common adverse reactions to the use of Saxenda, the frequency of which was higher than in the placebo group: nausea (42% of patients), vomiting (34%), diarrhea (22%), gastroenteritis (13%), dizziness (10 %). Over time, adverse events were observed significantly less frequently.
SAXENDA solution for injection. 6 mg/ml cartridge 3 ml No. 5 + spr.-pen
Pharmacodynamics
Mechanism of action
The active substance of Saxenda, liraglutide, is an analogue of human glucagon-like peptide-1 (GLP-1), produced by recombinant DNA biotechnology using the Saccharomyces cerevisiae strain, which has 97% amino acid sequence homology to endogenous human GLP-1. Liraglutide binds to and activates the GLP-1 receptor (GLP-1R). Liraglutide is resistant to metabolic degradation, its half-life from plasma after subcutaneous administration is 13 hours. The pharmacokinetic profile of liraglutide, which allows it to be administered to patients once a day, is the result of self-association, which results in delayed absorption of the drug, binding to plasma proteins, and resistance to dipeptidyl peptidase-4 (DPP-4) and neutral endopeptidase (NEP).
GLP-1 is a physiological regulator of appetite and food consumption. GLP-1R is found in several brain regions involved in appetite regulation. In animal studies, administration of liraglutide resulted in its uptake into specific areas of the brain, including the hypothalamus, where liraglutide, through specific activation of GLP-1R, increased satiety signals and decreased hunger signals, thereby leading to weight loss.
Liraglutide reduces body weight in humans primarily by reducing adipose tissue mass. Reducing body weight occurs by reducing food consumption. Liraglutide does not increase 24-hour energy expenditure. Liraglutide regulates appetite by increasing feelings of fullness and satiety, while reducing feelings of hunger and reducing expected food intake.
Liraglutide stimulates insulin secretion and reduces excessive glucagon secretion in a glucose-dependent manner, and also improves pancreatic beta cell function, which leads to a decrease in fasting and postprandial glucose concentrations. The mechanism for lowering glucose concentrations also involves a slight delay in gastric emptying.
Pharmacodynamics
In long-term clinical studies involving overweight or obese patients, the use of Saxenda in combination with a low-calorie diet and increased physical activity led to significant weight loss.
Effects on appetite, calorie intake, energy expenditure, gastric emptying, and fasting and postprandial glucose concentrations
The pharmacodynamic effects of liraglutide were studied in a five-week study involving 49 obese patients (body mass index (BMI) 30-40 kg/m2) without diabetes.
Appetite, calorie intake and energy expenditure
It is believed that weight loss with Saxenda is associated with regulation of appetite and calorie intake. Appetite was assessed before and for 5 hours after a standard breakfast, and ad libitum food intake was assessed during the subsequent lunch. Saxenda increased postprandial satiety and fullness and decreased hunger, estimated food intake, and ad libitum food intake compared with placebo. When assessed using a respiratory chamber, no treatment-related increase in 24-hour energy expenditure was noted.
Gastric emptying
The use of Saxenda led to a slight delay in gastric emptying during the first hour after eating, resulting in a decrease in the rate of increase in concentration, as well as the total concentration of blood glucose after eating.
Concentrations of glucose, insulin and glucagon on an empty stomach and after meals
The concentrations of glucose, insulin and glucagon on an empty stomach and after a meal were assessed before and within 5 hours after a standardized meal. Compared with placebo, Saxenda reduced fasting and postprandial blood glucose concentrations (AUC0-60 min) during the first hour after meals, and also reduced 5-hour glucose AUC and rising glucose concentrations (AUC0-300 min) - In addition , Saxenda reduced postprandial concentrations of glucagon (AUC0-300 min) and insulin (AUC0-60 min) and increasing insulin concentrations (iAUC0-60 min) after meals compared to placebo.
Fasting and rising concentrations of glucose and insulin were also assessed during an oral glucose tolerance test (OGTT) with 75 g glucose before and after 1 year of therapy in 3731 obese patients with and without impaired glucose tolerance. Compared with placebo, Saxenda reduced fasting and rising glucose concentrations. The effect was more pronounced in patients with impaired glucose tolerance. In addition, Saxenda decreased fasting insulin concentrations and increased cumulative insulin concentrations compared to placebo.
Effect on fasting and rising glucose concentrations in patients with type 2 diabetes mellitus who are overweight or obese
Saxenda reduced fasting glucose concentrations and mean rising postprandial glucose concentrations (90 minutes after a meal, average for 3 meals per day) compared to placebo.
Pancreatic beta cell function
In clinical studies of up to one year with Saxenda in overweight or obese patients with or without diabetes mellitus, improvement and preservation of pancreatic beta cell function was demonstrated using measurements such as the homeostatic beta cell function assessment model. cells (HOMA-B) and the ratio of proinsulin and insulin concentrations.
Clinical efficacy and safety
The effectiveness and safety of Saxenda for long-term weight loss in combination with a low-calorie diet and increased physical activity was studied in 4 randomized, double-blind, placebo-controlled studies (3 studies lasting 56 weeks and 1 study lasting 32 weeks). The studies included a total of 5358 patients from 4 different populations: 1) patients with obesity or overweight and one of the following conditions/diseases: impaired glucose tolerance, hypertension, dyslipidemia, 2) patients with obesity or overweight with insufficiently controlled type 2 diabetes mellitus (HbA1c value in the range of 7-10%), before the start of the study, to correct HbA1c in these patients, the following were used: diet and exercise, metformin, sulfonylureas, glitazone alone or in any combination, 3) patients obese patients with moderate or severe obstructive apnea, 4) obese or overweight patients with concomitant hypertension or dyslipidemia who have achieved a weight loss of at least 5% using a low-calorie diet.
Body mass
Greater weight loss was achieved in obese/overweight patients treated with Saxenda compared with placebo in all study groups, including those with or without impaired glucose tolerance, type 2 diabetes mellitus, and moderate or severe obstructive apnea. In Study 1 (obese and overweight patients with or without impaired glucose tolerance), weight loss was 8.0% in patients receiving Saxenda compared to 2.6% in the placebo group. In Study 2 (obese and overweight patients with type 2 diabetes), weight loss was 5.9% in patients receiving Saxenda, compared with 2.0% in the placebo group. In Study 3 (obese and overweight patients with moderate to severe obstructive apnea), weight loss was 5.7% in patients receiving Saxenda, compared with 1.6% in the placebo group. In Study 4 (obese and overweight patients after a previous weight loss of at least 5%), further weight loss was 6.3% in patients receiving Saxenda, compared with 0.2% in the placebo group. In Study 4, more patients maintained the weight loss achieved before treatment with Saxenda compared to placebo (81.4% and 48.9%, respectively). In addition, in all study populations, a greater proportion of patients receiving Saxenda achieved weight loss of at least 5% and greater than 10% compared with patients receiving placebo.
In study No. 1 (obese and overweight patients with or without impaired glucose tolerance), a decrease in body weight of at least 5% at 56 weeks of therapy was observed in 63.5% of patients receiving Saxenda, compared with 26. 6% in the placebo group. The proportion of patients achieving greater than 10% weight loss at week 56 was 32.8% in the Saxenda group compared with 10.1% in the placebo group. Overall, weight loss occurred in approximately 92% of patients receiving Saxenda, compared with approximately 65% in the placebo group.
Weight loss after 12 weeks of therapy with Saxenda
Early responders were defined as those who achieved a weight loss of at least 5% after 12 weeks of therapy (4 weeks of escalation and 12 weeks of 3 mg therapy).
In two studies (obese or overweight patients without and with type 2 diabetes), 67.5% and 50.4% of patients achieved a weight loss of at least 5% after 12 weeks of therapy. With continued therapy with Saxenda (up to 1 year), 86.2% of these patients achieved a reduction in body weight of at least 5% and 51% of at least 10%. The mean weight loss in these patients who completed the study was 11.2% from baseline. In patients who achieved a weight loss of less than 5% after 12 weeks of 3 mg therapy and completed the study (1 year), the mean weight loss was 3.8%.
Glycemic control
Treatment with Saxenda significantly improved glycemic parameters in subpopulations with normoglycemia, impaired glucose tolerance (mean reduction in HbA1c - 0.3%) and type 2 diabetes mellitus (mean reduction in HbA1c - 1.3%) compared with placebo (mean reduction in HbA1c - 0.1% and - 0.4%, respectively). In a study of patients with impaired glucose tolerance, type 2 diabetes developed in fewer patients receiving Saxenda compared to the placebo group (0.2% and 1.1%, respectively). More patients with impaired glucose tolerance experienced reversal of this condition compared to the placebo group (69.2% and 32.7%, respectively).
In a study of patients with type 2 diabetes, 69.2% and 56.5% of patients receiving Saxenda achieved target HbA1c <.7% and <.6.5%, respectively, compared with 27.2% and 15.0% in patients receiving placebo.
Cardiometabolic parameters
In a study of obese or overweight patients with or without impaired glucose tolerance, significant reductions in systolic blood pressure (4.3 points versus 1.5 points) and diastolic blood pressure (2.7 points) were observed with Saxenda. vs. 1.8 points), waist circumference (8.2 cm vs. 4.0 cm), and a significant change in fasting lipid concentrations (3.2% vs. 0.9% decrease in total cholesterol, 3.1% decrease in low-density lipoprotein % vs. 0.7%, 2.3% vs. 0.5% increase in high-density lipoprotein, 13.6% vs. 4.8% decrease in triglycerides compared to placebo.
Apnea-hypnosis index
When using Saxenda, there was a significant reduction in the severity of obstructive apnea compared with placebo, which was assessed by a decrease in the apnea-hypnea index (AHI) by 12.2 cases per hour and 6.1 cases per hour, respectively.
Immunogenicity
Given the potential immunogenic properties of protein and peptide drugs, patients may develop antibodies to liraglutide after therapy with Saxenda. In clinical studies, 2.5% of patients receiving Saxenda developed antibodies to liraglutide. The formation of antibodies did not lead to a decrease in the effectiveness of Saxenda.
Assessment of cardiovascular events
Major adverse cardiovascular events (MACE) were assessed by a panel of external independent experts and defined as non-fatal myocardial infarction, non-fatal stroke and death due to cardiovascular disease. In all long-term clinical studies using Saxenda, 6 MACEs were observed in patients receiving Saxenda and 10 MACEs in patients receiving placebo. The risk ratio and 95% CI when comparing Saxenda with placebo was 0.31, 0.10, 0.92. In phase 3 clinical studies, an average increase in heart rate (HR) of 2.5 beats per minute (range 1.6 to 3.6 beats per minute in individual studies) was observed in patients receiving Saxenda. The greatest increase in heart rate was observed after 6 weeks of therapy. This increase was reversible and disappeared after discontinuation of liraglutide therapy.
Patient Assessment Results
Compared with placebo, Saxenda improved patient-reported scores on selected measures. There was a significant improvement in overall scores on the Inventory of Weight on Quality of Life-Lite (IWQoL-Lite) and on all scales of the SF-36 Quality of Life Questionnaire, indicating a positive impact on the physical and psychological components of quality of life.
Preclinical Safety Data
Preclinical safety
, repeated dose toxicity and genotoxicity studies did not indicate any hazard to humans.
Two-year carcinogenicity studies in rats and mice identified thyroid C-cell tumors that were not fatal. The nontoxic dose (NOAEL) in rats has not been established. Monkeys treated for 20 months did not develop these tumors. The results obtained from rodent studies are due to the fact that rodents are particularly sensitive to the GLP-1 receptor-mediated non-genotoxic specific mechanism. The significance of the data obtained for humans is low, but cannot be completely excluded. The appearance of other neoplasms associated with the therapy was not noted.
In animal studies, no direct adverse effect of the drug on fertility was detected, but a slight increase in the incidence of early embryonic death was noted at the highest doses of the drug. Administration of liraglutide in the middle of the gestational period caused a decrease in maternal body weight and fetal growth with an unexplored effect on the ribs in rats, and in rabbits - deviations in the skeletal structure. Neonatal growth was reduced in rats during liraglutide treatment, and this reduction was maintained after cessation of breastfeeding in the high-dose group. It is not known whether this decrease in growth of newborn rats is due to a decrease in maternal caloric intake or a direct effect of GLP-1 on the fetus/newborns.